Adhesion Describes Water's Attraction to
Water molecules being attracted to other water molecules is called cohesion. Cohesion refers to the attraction of molecules for other molecules of the same kind while adhesion is the attraction of molecules of one kind for molecules of a different kind.

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey
Water is attracted to other substances.
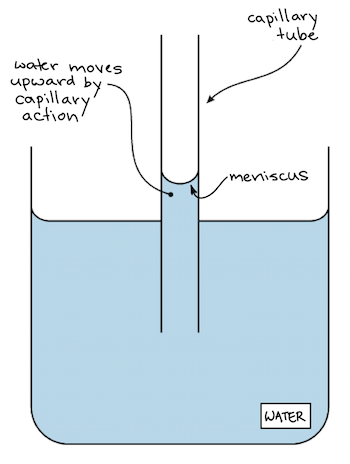
. Another example is to dip a cloth in water and see how water goes up into. Surface tension is the property of water in which. Adhesion and cohesion are water properties that affect every water molecule on Earth and also the interaction of water molecules with molecules of other substances.
Waters attraction to other substances. Adhesion refers to the tendency of water molecules to be attracted or stick to other substances. Cohesion of water molecules at the surface of a body of water.
Adhesion refers to the tendency of water molecules to be attracted or stick to other substances. Adhesion is the attraction of molecules of one kind for molecules of a different kind and it can be quite strong for water. For example put a glass.
Water molecules at the surface tend to stick together. Login is required in order to view results and track your progress. This type of bond results from an electronegative atom bonded to a hydrogen attracted to another electronegative atom.
The mere exposure effect provides one possible explanation for why _____ increases attraction. Molecular condition in which one end has a slightly positive charge and the other a slightly negative charge. Adhesion and cohesion are water properties that affect every water molecule on earth and also the interaction of water molecules with molecules of other substances.
Jupiter is 317 times more massive than the earth. Two or more elements joined together to create a new substance. Their attraction to other substances is adhesion.
This is a result of the covalent bond between the two hydrogen atoms and the one oxygen atom in the water molecule. This is a result of the covalent bond between the two hydrogen atoms and the one oxygen atom in the water molecule. Tube into water and see how water rises slightly in the tube.
Cohesion is the attraction between like molecules such as water to water while adhesion is the attraction between unlike molecules such as water to any surface. Surface tension _____causes water to spread out is the ability of water to dissolve a substance creates a thin elastic layer of hydrogen atoms is caused by. The word that best describes when water is attracted to other substances is adhesion.
Attraction between different. Attraction to other substances. Adhesion and cohesion are both water properties.
Essentially cohesion and adhesion are the stickiness that water molecules have for each other and for other substances. A simple definition of adhesion in water would be that water sticks to things. Adhesion describes waters attraction to _____.
Adhesion is the attraction of molecules of one kind for molecules of a different kind and it can be quite strong for water especially with other molecules bearing positive or negative charges. Other water molecules b. This is occurring because of.
Please continue with your Google account. Cohesion refers to the attraction of molecules for other molecules of the same kind while adhesion is the attraction of molecules of one kind for molecules of a different kind. Cohesion describes waters _____attraction to other substances attraction to itself ability to dissolve most substances rate of cooling and heating Weegy.
The property where water molecules are attracted to other water molecules. Cohesion surface tension and adhesion are the properties of water molecules that _____. 15 Questions Show answers.
Its gravitational attraction is _____ earths. Attraction to other water molecules. This is because the water molecules are more strongly attracted to the sides of the tube than to each other.
The attraction of water molecules to other water molecules. Adhesion describes waters attraction to. Essentially cohesion and adhesion are the stickiness that water molecules have for each other and for.
Water tends to be see-through. The word that best describes when water is attracted to other substances is adhesion. Water likes to stick to itself but under certain circumstances it actually prefers to stick to other types of molecules.
Water is attracted to water Adhesion. Rather than staying in one area the water. What term describes when water is attracted to other substances.
Adhesion is the attraction of molecules of one kind for molecules of a different kind and it can be quite strong for water especially with other molecules bearing positive or negative charges. What word describes when water is attracted to water. Answered by administrator 23062021.
Adhesion describes waters attraction to other water molecules. Refer to the picture of water sticking to a leaf. An example of this can be seen when pouring water into a shallow dish.
Waters attraction to other water molecules. Water is attracted to other substances. Other water molecules other substances hydrogen molecules or acids and bases Weegy.
Cohesion describes waters attraction to itself. Molecular condition in which one end has a slightly positive charge and the other a slightly negative charge. Two or more elements joined together to create a new substance.
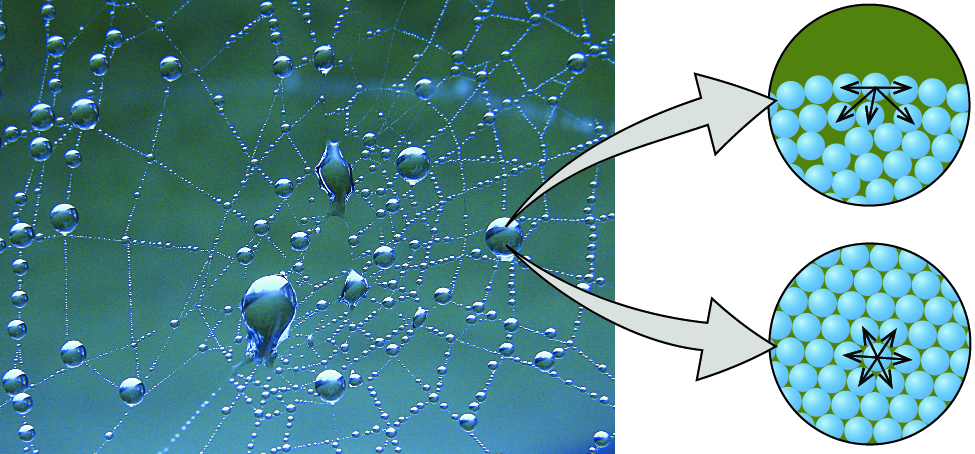
Cohesion And Adhesion Of Water Article Khan Academy

Cohesion And Adhesion Of Water Article Khan Academy

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey
Comments
Post a Comment